Adsorption kinetics and isotherms of penconazole, cyproconazole and epoxiconazole with chitosan
DOI:
https://doi.org/10.24054/bistua.v19i2.1100Keywords:
fungicides, adsorption, chitosan, isothermsAbstract
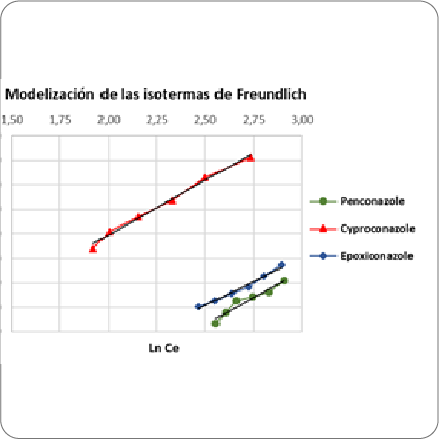
In the search for alternative technologies that can mitigate the contamination problem in water sources, mainly those destined for human consumption, the chitosan powder efficiency for the pesticide removal has been evaluated. In adsorption processes there is a wide spectrum of physical and chemical mechanisms, which normally occur in consecutive stages such as the diffusion of the adsorbate through the fluid film to the adsorbent material, diffusion through the pores and subsequent reaction of adsorption. In this process, it was observed that each analyte, depending on its physicochemical and / or structural properties, presented different adsorptions, which was confirmed observing to the variation in pesticide removal percentages using the same adsorbents quantities. The optimization of the removal variables allowed to determine that the maximization values for most analytes percentage removal are similar and close to the maximum values of contact time and amount of adsorbent. The adsorption kinetics study allowed to establish the equilibrium time, which was 180 minutes for the analytes, and the data modeling wich responded to a pseudo-second order kinetics. Regarding to the adsorption isotherms' modeling, the best fit model is Freundlich's. Faced with this complex issue, the evaluation of pesticide removal through technological processes becomes relevant in order to maintain the water resources availability with adequate quality.
Downloads
References
Lhomme L., Brosillon S., Wolbert D. Photocatalytic degradation of a triazole pesticide, cyproconazole, in water. Journal of Photochemistry and Photobiology A: Chemistry, 188 (2007) 34–42.
Hetz E., Saavedra M. Venegas A., López M. Ventanas de aplicación de plaguicidas en huertos de arándanos (Vaccinium sp.) de la zona de Los Ángeles, Chile. Agricultura Técnica 64(4)(2004) 375-387.
Sasal C., Andriulo A.E., Wilson M.G., Portela S.I. Pérdidas de Glifosato por Drenaje y Escurrimiento y Riesgo de Contaminación de Aguas. En Aspectos Ambientales del Uso de Glifosato. Ed. Instituto Nacional de Tecnología Agropecuaria (2010) 101-114.
Tang X., Zhu B., Katou H. A review of rapid transport of pesticides from sloping farmland to surface waters: Processes and mitigation strategies. Journal of Environmental Sciences, 24 (2012) 351 – 361
Ronco A.E. Impacto de plaguicidas en ambientes acuáticos pampeanos: Integración de estudios químico ecotoxicológicos en experimentos de campo y laboratorio, con especial énfasis al caso del glifosato. En Aspectos Ambientales del Uso de Glifosato. Ed. Instituto Nacional de Tecnología Agropecuaria (2010) 85-94.
Andrade A., Stigter T. Multi-method assessment of nitrate and pesticide contamination in shallow alluvial groundwater as a function of hydrogeological setting and land use. Agricultural Water Management 96 (2009) 1751–1765.
Menezes Filho A., Neves dos Santos F., Pereira, P. Development, validation and application of a method based on DI-SPME and GC–MS for determination of pesticides of different chemical groups in surface and groundwater samples. Microchemical Journal 96 (2010) 139-145.
Singer H., Jaus S., Hanke I., Lück A., Hollender J., Alder A. Determination of biocides and pesticides by on-line solid phase extraction coupled with mass spectrometry and their behaviour in wastewater and surface water. Environmental Pollution 158 (2010) 3054-3064.
Foo K.Y., Hameed B.H. Detoxification of pesticide waste via activated carbon adsorption process. Journal of Hazardous Materials, 175 (2010) 1 – 11.
Reungoat J., Macova M., Escher B.I., Carswell S., Mueller J.F., Keller J. Removal of micropollutants and reduction of biological activity in a full scale reclamation plant using ozonation and activated carbon filtration. Water Research, 44 (2010) 625 – 637.
Delgado L. F., Charles P., Glucina K., Morlay C. The removal of endocrine disrupting compounds, pharmaceutically activated compounds and cyanobacterial toxins during drinking water preparation using activated carbon - A review. Science of the Total Environment, 435-436 (2012) 509–525.
Lebrón Orellana R. Adsorción de Paraquat con Polímeros Naturales. Escola Politécnica Superior d’Enginyeria de Vilanova i la Geltrú. Universitat Politécnica de Catalunya. (2007).
Warren L., Mc Cabe J. Operaciones Unitarias en Ingeniería Química. McGraw-Hill-Interamericana de México. (2007) ISBN 9789701061749.
Soni U., Bajpai J., Singh S.K., Bajpai A.K. Evaluation of chitosan-carbon based biocomposite for efficient removal of phenols from aqueous solutions. Journal of Water Process Engineering, 16 (2017) 56–63.
Gupta V.K., Gupta B., Rastogi A., Agarwal S., Nayak, A. Pesticides removal from waste water by activated carbon prepared from waste rubber tire. Water Research, 45 (2011) 4047 – 4055.
Jusoh A., Hartini W.J.H., Ali N., Endut, A. Study on the removal of pesticide in agricultural run off by granular activated carbon. Bioresource Technology, 102 (2011) 5312 - 5318.
Tapia H.N., Cabrejos G.J., Rojas P.N., Chasquibol N.S., Yarango A.R., Torres D.F., Becerra V.E. Perlas de Quitosano con Partículas Magnéticas y su Aplicación en la Adsorción de Iones de Cu (II). Rev. Per. Quím. Ing. Quím., 15, N.º 1 (2012) 54-58.
Saad A.H.A., Azzam A.M., El-Wakeel S.T., Mostafa B.B., El-latif M.B.A. Removal of toxic metal ions from wastewater using ZnO@Chitosan coreshell nanocomposite. Environmental Nanotechnology, Monitoring & Management, 9 (2018) 67–75.
Hosseinzadeh H., Ramin S. Effective removal of copper from aqueous solutions by modified magnetic chitosan/graphene oxide nanocomposites. International Journal of Biological Macromolecules, 113 (2018) 859–868.
Naseeruteen F., Hamid N.S.A., Mohd Suah F.B., Wan Ngah W.S., Mehamod F.S. Adsorption of malachite green from aqueous solution by using novel chitosan ionic liquid beads. International Journal of Biological Macromolecules, 107 (2018) 1270–1277.
Shankar A., Kongot M., Kumar Saini V., Kumar, A. Removal of pentachlorophenol pesticide from aqueous solutions using modified chitosan. Arabian Journal of Chemistry. Article in press. (2018).
Lárez Velásquez C. Quitina y quitosano: materiales del pasado para el presente y el futuro. Avances en Química, 1(2) (2006) 15-21.
Mármol Z., Paéz G., Rincón M., Araujo K., Aiello C., Chandler C., Gutiérrez E. Quitina y Quitosano polímeros amigables. Una revisión de sus aplicaciones. Revista Tecnocientífica URU (Universidad Rafael Urdaneta), N° 1 (2011) 53-58.
Dhaouadi A., Monser L., Adhoum N. Removal of rotenone insecticide by adsorption onto chemically modified activated carbons. Journal of Hazardous Materials, 181 (2010) 692 – 699.
Lu L.C., Wang C.I., Sye W.F. Applications of chitosan beads and porous crab shell powder for the removal of 17 organochlorine pesticides (OCPs) in water solution. Carbohydrate Polymers, 83 (2011) 1984-1989.
Agostini de Moraes M., Sgarbi Cocenza D., da Cruz Vasconcellos F., Fernandes Fraceto L., Masumi Beppu, M. Chitosan and alginate biopolymer membranes for remediation of contaminated water with herbicides. Journal of Environmental Management, 131 (2013) 222-227.
El Harmoudi H., El Gaini L., Daoudi E., Rhazi M., Boughaleb Y., El Mhammedi M.A., Migalska-Zalas A., Bakasse, M. Removal of 2,4-D from aqueous solutions by adsorption processes using two biopolymers: chitin and chitosan and their optical properties. Optical Materials, 36 (2014) 1471-1477.
Medina M.B, Munitz M.S., Resnik S.L. Validation and expanded uncertainty determination of pesticides in water; and their survey on paddy rice irrigation water from Argentina. Journal of Environmental Science and Health, Part B 55(11) (2020) 983-989.
Kaur R., Goyal D., Agnihotri S. Chitosan/PVA silver nanocomposite for butachlor removal: Fabrication, characterization, adsorption mechanism and isotherms. Carbohydrate Polymers, 262 (2021) 117906.
Moustafa M., Abu-Saied M.A. , Taha T., Elnouby M., El-shafeey M., Alshehri A.G., Alamri S., Shati A., Alrumman S., Alghamdii H., Al-Khatani M. Chitosan functionalized AgNPs for efficient removal of Imidacloprid pesticide through a pressure-free design. International Journal of Biological Macromolecules, 168 (2021) 116–123.
Borja-Urzola A., García-Gómez R., Bernal-González M., Durán-Domínguez-de-Bazúa M. Chitosan-calcite from shrimp residues: A low-cost adsorbent for three triazines removal from aqueous media. Materials Today Communications, 26 (2021) 102131.
Keshvardoostchokami M., Majidi M., Zamani A., Liu B. Adsorption of phenol on environmentally friendly Fe3O4/chitosan/zeolitic imidazolate framework-8 nanocomposite: Optimization by experimental design methodology. Journal of Molecular Liquids, 323 (2021) 115064.
Solano R.A., De León L.D., De Ávila G., Herrera A.P. Polycyclic aromatic hydrocarbons (PAHs) adsorption from aqueous solution using chitosan beads modified with thiourea, TiO2 and Fe3O4 nanoparticles. Environmental Technology & Innovation, 21(2021) 101378.
Shankar A., Kongot M., Kumar Saini V., Kumar A. Removal of pentachlorophenol pesticide from aqueous solutions using modified chitosan. Arabian Journal of Chemistry, 13 (2020) 1821–1830.
Additional Files
Published
Versions
- 2021-12-22 (6)
- 2022-02-05 (5)
- 2021-12-21 (4)
- 2021-12-21 (3)
How to Cite
Issue
Section
License
Copyright (c) 2021 © Autores; Licencia Universidad de Pamplona.

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
© Autores; Licencia Universidad de Pamplona





