Actividad antagónica in vitro de cepas nativas de Trichoderma contra Botrytis sp
In vitro antagonistic activity of native Trichoderma strains against Botrytis sp
DOI:
https://doi.org/10.24054/bistua.v23i1.3660Palabras clave:
Trichoderma, Control biológico, Antagonismo, BotrytisResumen
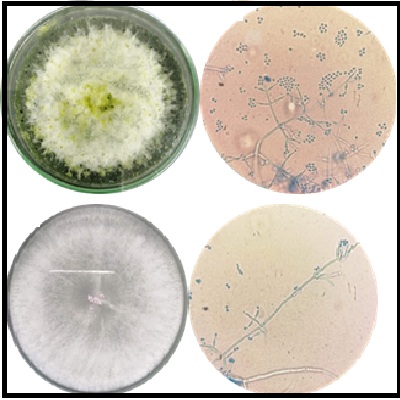
Botrytis cinerea es un agente fitopatógeno que afecta una enorme variedad de plantas, siendo el responsable de graves pérdidas económicas en más de 200 especies de cultivos en todo el mundo. Aunque el uso de fungicidas es la estrategia más usada para su control, la plasticidad genética de este hongo le permite desarrollar resistencia. Por tanto, el control biológico ha ganado terreno como un enfoque alternativo o complementario a los fungicidas. A este respecto, el género Trichoderma constituye el grupo más estudiado y con mayor potencial para el control biológico de B. cinerea. Por tanto, la búsqueda y estudio de cepas nativas de Trichoderma con potencial biocontrolador es una línea de interés para el sector agrícola local y regional. En este trabajo reportamos el estudio in vitro de la capacidad biocontroladora de un producto comercial, en comparación con el potencial antagonista de dos cepas autóctonas aisladas de suelos de zonas agrícolas de los municipios de Pamplona (TN01) y Bucarasica (TN02), en Norte de Santander. La caracterización morfológica determinó que ambas cepas se ubican taxonómicamente en el clado Viride del género Trichoderma. Mediante la técnica de cultivo dual se determinó la velocidad de crecimiento y el porcentaje de inhibición del crecimiento radial (PICR) de los antagonistas. Los resultados indicaron que la cepa con mayor potencial para ser usada como controlador biológico de Botrytis spp., fue TN01. Esta cepa mostró la mayor velocidad de crecimiento apical y consiguió un PICR del 80% al final del experimento, superando lo conseguido por el producto comercial de referencia. Por su parte, la cepa TN02 mostró una menor capacidad antagónica con un PICR de 55% después de 12 días. Lo anterior, nos permite sugerir el uso de la cepa autóctona Trichoderma TN01 para ser ensayada en condiciones de cultivo.
Descargas
Referencias
Aldana Bohórquez, S.M. and García Rico, R.O. 2019. Efecto de diferentes condiciones de estrés sobre el crecimiento vegetativo del hongo filamentoso Acremonium chrysogenum. BISTUA REVISTA DE LA FACULTAD DE CIENCIAS BASICAS. 17, 2 (Aug. 2019), 182–195. https://doi.org/10.24054/bistua.v17i2.248
Azevedo, D.M.Q., Martins, S.D.S., Guterres, D.C., Martins, M.D., Araújo, L., Guimarães, L.M.S., Alfenas, A.C., Furtado, G.Q., 2020. Diversity, prevalence and phylogenetic positioning of Botrytis species in Brazil. Fungal Biol. 124, 940–957. https://doi.org/10.1016/j.funbio.2020.08.002
Brankica, T., Delibasic, G., Milivojevic, J., Nikolic, M., 2009. Characterization of Botrytis cinerea isolates from small fruits and grapevine in Serbia. Arch. Biol. Sci. 61. https://doi.org/10.2298/ABS0903419T
Cheung, N., Tian, L., Liu, X., Li, X., 2020. The Destructive Fungal Pathogen Botrytis cinerea-Insights from Genes Studied with Mutant Analysis. Pathog. Basel Switz. 9, E923. https://doi.org/10.3390/pathogens9110923
Crous, P.W., Verkley, G.J.M., Groenewald, J.Z., Samson, R.A., 2009. Fungal Biodiversity. CBS Laboratory Manual Series. Westerdijk Fungal Biodiversity Institute
Cruz, A.F., Barka, G.D., Sylla, J., Reineke, A., 2018. Biocontrol of strawberry fruit infected by Botrytis cinerea: Effects on the microbial communities on fruit assessed by next-generation sequencing. J. Phytopathol. 166, 403–411. https://doi.org/10.1111/jph.12700
Debnath, S., Chakraborty, G., Dutta, S.S., Chaudhury, S.R., Das, P., Saha, A.K., 2020. Potential of Trichoderma species as biofertilizer and biological control on Oryza sativa L. cultivation. Biotecnol. Veg. 20, 1–16.
Díaz de la Osa A, Almenares Casanova M, Fernández Millares B, Aguado Casas ME, Rojas L, Zeilinger S, Hernández-Rodríguez A. Secondary metabolites and extracellular proteases contribute to the antagonistic action of indigenous Trichoderma strains against Botrytis cinerea. Fungal Biol. 2025 Feb;129(1):101530. doi: 10.1016/j.funbio.2024.101530. Epub 2024 Dec 31. PMID: 39826978.
Elías, R., Arcos, O., Arbeláez, G., 1993. Estudio del antagonismo de algunas especies de Trichoderma aisladas de suelos colombianos en el control de Fusarium oxysporum y Rhizoctonia solani. Agron. Colomb. 10, 52–61.
Fernandes, V.C., Maia, M.L., Correia Sá, L., Sousa, S., Paíga, P., Vera, J.L., Domingues, V.F., Delerue-Matos, C., 2021. Extraction Procedures and Chromatography of Pesticides Residues in Strawberries, in: Inamuddin, Ahamed, M.I., Lichtfouse, E. (Eds.), Sustainable Agriculture Reviews 47: Pesticide Occurrence, Analysis and Remediation Vol. 1 Biological Systems, Sustainable Agriculture Reviews. Springer International Publishing, Cham, pp. 167–201. https://doi.org/10.1007/978-3-030-54712-7_5
Fernández, O, D., Grabke, A., Li, X., Schnabel, G., 2015. Independent Emergence of Resistance to Seven Chemical Classes of Fungicides in Botrytis cinerea. Phytopathology® 105, 424–432. https://doi.org/10.1094/PHYTO-06-14-0161-R
Fernández, O, D., Li, X.P., Wang, F., Schnabel, G., 2012. First Report of Gray Mold of Strawberry Caused by Botrytis caroliniana in North Carolina. Plant Dis. 96, 914–914. https://doi.org/10.1094/PDIS-12-11-1018-PDN
García-Rico, R.O. and Fierro, F., 2017. Papel de las subunidades alfa de proteínas G en los procesos morfogénicos de hongos filamentosos de la división Ascomycota. Rev. Iberoam. Micol. 34, 1–9. https://doi.org/10.1016/j.riam.2016.06.005
Harman, G.E., Kubicek, C.P., 2002. Trichoderma And Gliocladium. Volume 1: Basic Biology, Taxonomy and Genetics. CRC Press.
He, L., Cui, K., Li, T., Song, Y., Liu, N., Mu, W., Liu, F., 2020. Evolution of the Resistance of Botrytis cinerea to Carbendazim and the Current Efficacy of Carbendazim Against Gray Mold After Long-Term Discontinuation. Plant Dis. 104, 1647–1653. https://doi.org/10.1094/PDIS-11-19-2457-RE
Isaza, L., Zuluaga, Y.P., Marulanda, M.L., 2019. Morphological, pathogenic and genetic diversity of Botrytis cinerea Pers. in blackberry cultivations in Colombia. Rev. Bras. Frutic. 41. https://doi.org/10.1590/0100-29452019490
Jaklitsch, W.M., 2009. European species of Hypocrea Part I. The green-spored species. Stud. Mycol., European species of Part I. The green-spored species 63, 1–91. https://doi.org/10.3114/sim.2009.63.01
Jaklitsch, W.M., Voglmayr, H., 2015. Biodiversity of Trichoderma (Hypocreaceae) in Southern Europe and Macaronesia. Stud. Mycol., Hypocrealean lineages of industrial and phytopathological importance 80, 1–87. https://doi.org/10.1016/j.simyco.2014.11.001
Kumar, Manish, Ashraf, S., 2017. Role of Trichoderma spp. as a Biocontrol Agent of Fungal Plant Pathogens, in: Kumar, V., Kumar, Manoj, Sharma, S., Prasad, R. (Eds.), Probiotics and Plant Health. Springer, Singapore, pp. 497–506. https://doi.org/10.1007/978-981-10-3473-2_23
Martinez Y, Ribera J, Schwarze FWMR, De France K. Biotechnological development of Trichoderma-based formulations for biological control. Appl Microbiol Biotechnol. 2023 Sep;107(18):5595-5612. doi: 10.1007/s00253-023-12687-x. Epub 2023 Jul 21. PMID: 37477696; PMCID: PMC10439859.
McGuire, A.V., Northfield, T.D., 2021. Identification and evaluation of endemic Metarhizium strains for biological control of banana rust thrips. Biol. Control 162, 104712. https://doi.org/10.1016/j.biocontrol.2021.104712
Meng, L., Mestdagh, H., Ameye, M., Audenaert, K., Höfte, M., Van Labeke, M.-C., 2020. Phenotypic Variation of Botrytis cinerea Isolates Is Influenced by Spectral Light Quality. Front. Plant Sci. 0. https://doi.org/10.3389/fpls.2020.01233
Mirzaei, S., Goltapeh, E.M., Shams-Bakhsh, M., Safaie, N., 2008. Identification of Botrytis spp. on Plants Grown in Iran. J. Phytopathol. 156, 21–28. https://doi.org/10.1111/j.1439-0434.2007.01317.x
Pei, Y., Tao, Q., Zheng, X., Li, Y., Sun, X., Li, Z., Qi, X., Xu, J., Zhang, M., Chen, H., Chang, X., Tang, H., Sui, L., Gong, G., 2019. Phenotypic and Genetic Characterization of Botrytis cinerea Population from Kiwifruit in Sichuan Province, China. Plant Dis. 103, 748–758. https://doi.org/10.1094/PDIS-04-18-0707-RE
Petrasch, S., Knapp, S.J., Kan, J.A.L. van, Blanco-Ulate, B., 2019. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 20, 877–892. https://doi.org/10.1111/mpp.12794
Qin, W.-T., Zhuang, W.-Y., 2016. Seven wood-inhabiting new species of the genus Trichoderma (Fungi, Ascomycota) in Viride clade. Sci. Rep. 6, 27074. https://doi.org/10.1038/srep27074
Samaniego-Fernández, L.M., Harouna, M., Corbea, O., Rondón-Castillo, A.J., Placeres-Espinosa, I., Samaniego-Fernández, L.M., Harouna, M., Corbea, O., Rondón-Castillo, A.J., Placeres-Espinosa, I., 2018. Aislamiento, identificación y evaluación de cepas autóctonas de Trichoderma spp. antagonistas de patógenos del suelo. Rev. Protección Veg. 33.
Samuels, G.J., Dodd, S.L., Lu, B.-S., Petrini, O., Schroers, H.-J., Druzhinina, I.S., 2006. The Trichoderma koningii aggregate species. Stud. Mycol. 56, 67–133. https://doi.org/10.3114/sim.2006.56.03
Shah, M.M., Afiya, H., 2019. Introductory Chapter: Identification and Isolation of Trichoderma spp. - Their Significance in Agriculture, Human Health, Industrial and Environmental Application, Trichoderma - The Most Widely Used Fungicide. IntechOpen. https://doi.org/10.5772/intechopen.83528
Torrent C, Gil-Durán C, Rojas-Aedo JF, Medina E, Vaca I, Castro P, García-Rico RO, Cotoras M, Mendoza L, Levicán G, Chávez R. Role of sfk1 Gene in the Filamentous Fungus Penicillium roqueforti. Front Microbiol. 2017 Dec 6;8:2424. doi: 10.3389/fmicb.2017.02424. PMID: 29270163; PMCID: PMC5723657.
Woo SL, Hermosa R, Lorito M, Monte E. Trichoderma: a multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat Rev Microbiol. 2023 May;21(5):312-326. doi: 10.1038/s41579-022-00819-5. Epub 2022 Nov 22. PMID: 36414835.
Zin, N.A., Badaluddin, N.A., 2020. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 65, 168–178. https://doi.org/10.1016/j.aoas.2020.09.003
Archivos adicionales
Publicado
Número
Sección
Licencia
Derechos de autor 2025 © Autores; Licencia Universidad de Pamplona

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
© Autores; Licencia Universidad de Pamplona.





